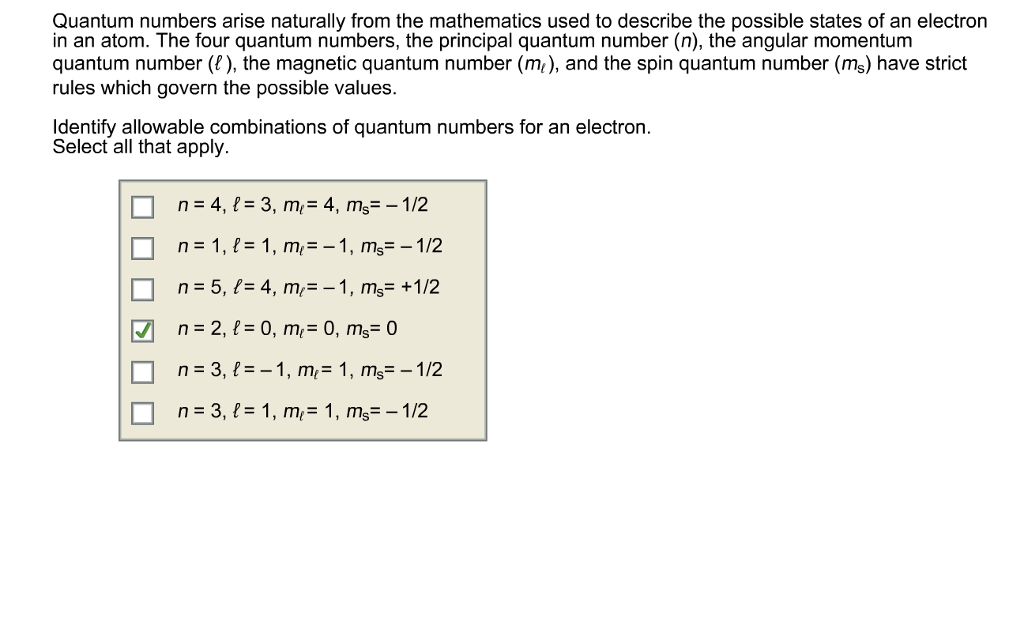
Spin Angular Momentum Quantum Number
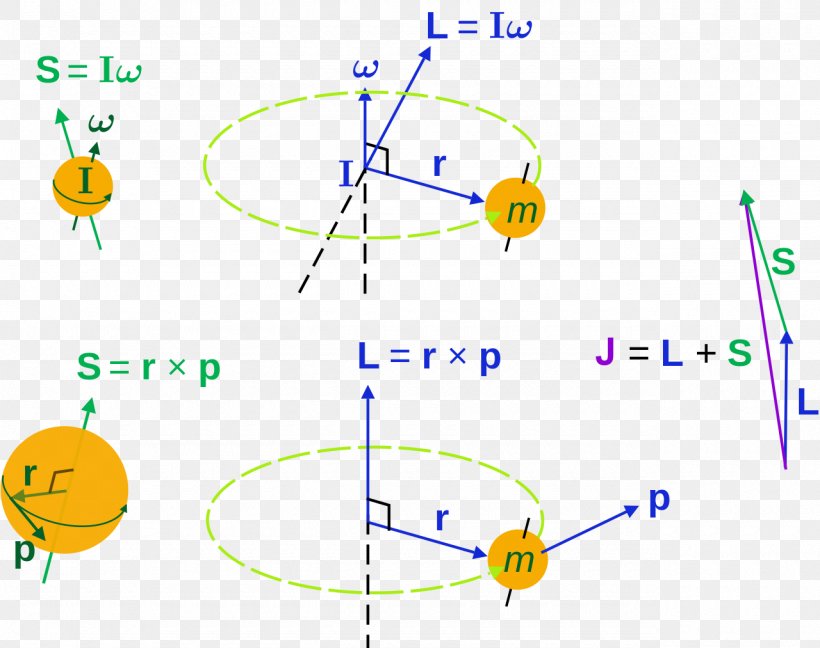
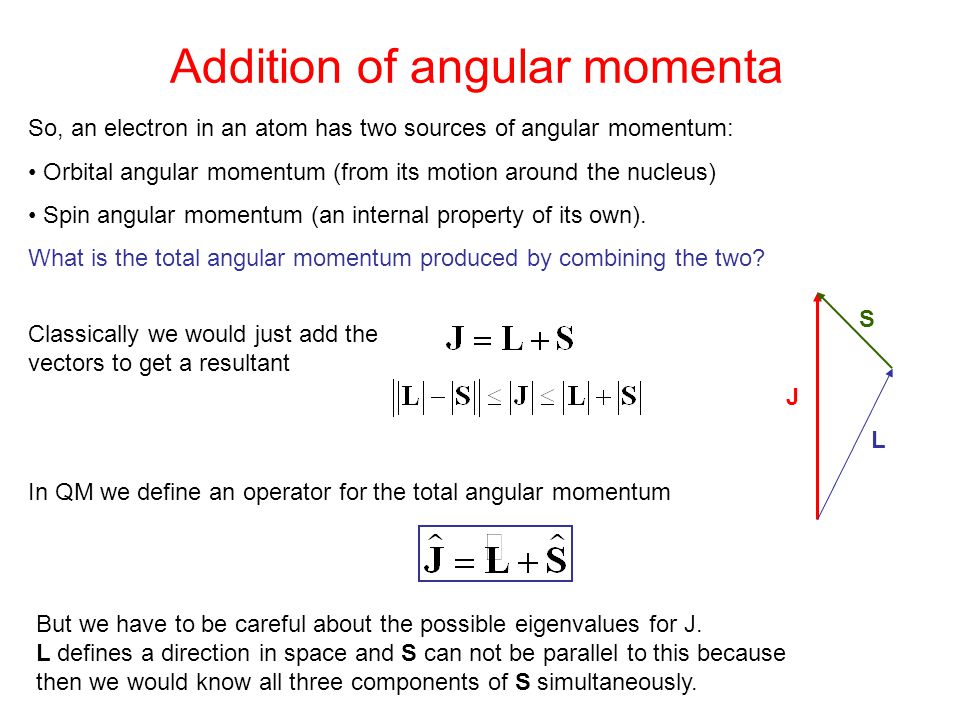
It is often required to add angular momentum from two (or more) sources together to get states of definite total angular momentum.

Spin angular momentum quantum number. Angular Momentum Quantum # (symbol) l. Small particles like protons, neutrons, and electrons are often shown to be spinning on an axis like a planet, but this simply cannot be the case. All right, our second quantum number is called the angular momentum quantum number.
An electron spins around an axis and has both angular momentum and orbital angular momentum. In other words, spin has no analogy in classical physics. The angular momentum quantum number can have positive values of zero to (n − 1).
S is the spin quantum number associated with the spin angular momentum, is Planck's reduced constant (Dirac's constant). The spin quantum number is known to arise from this intrinsic value of angular momentum. Gives information about energy level.
Principal quantum number (n):. Addition of angular momentum Problem:. An electron spin s = 1/2 is an intrinsic property of electrons.Electrons have intrinsic angular momentum characterized by quantum number 1/2.
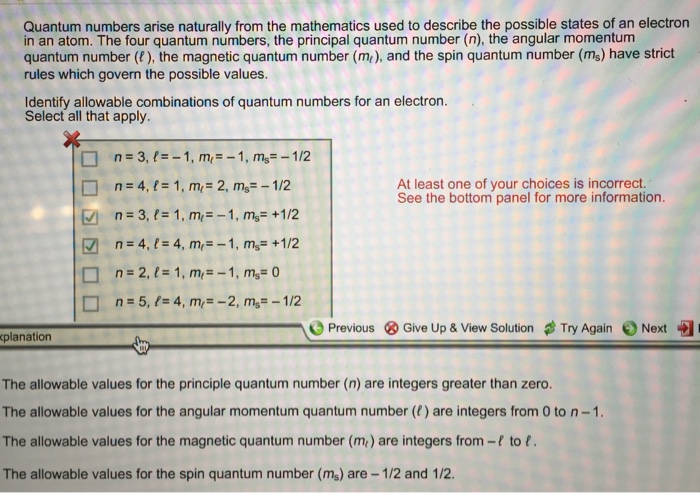
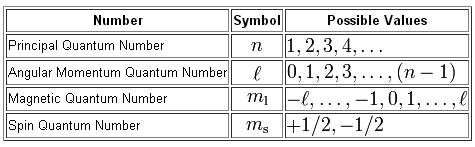
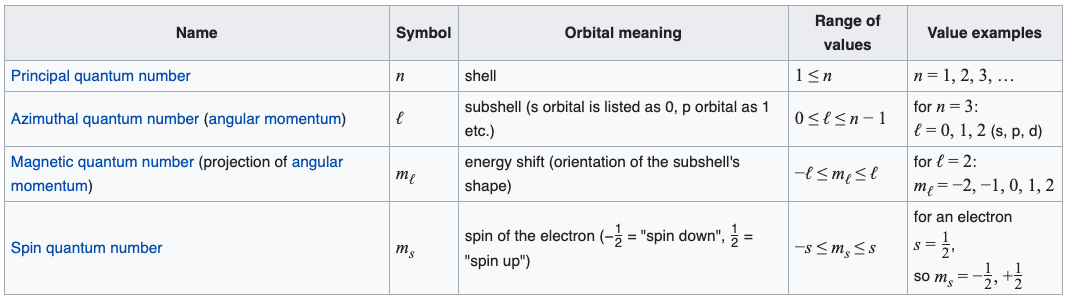
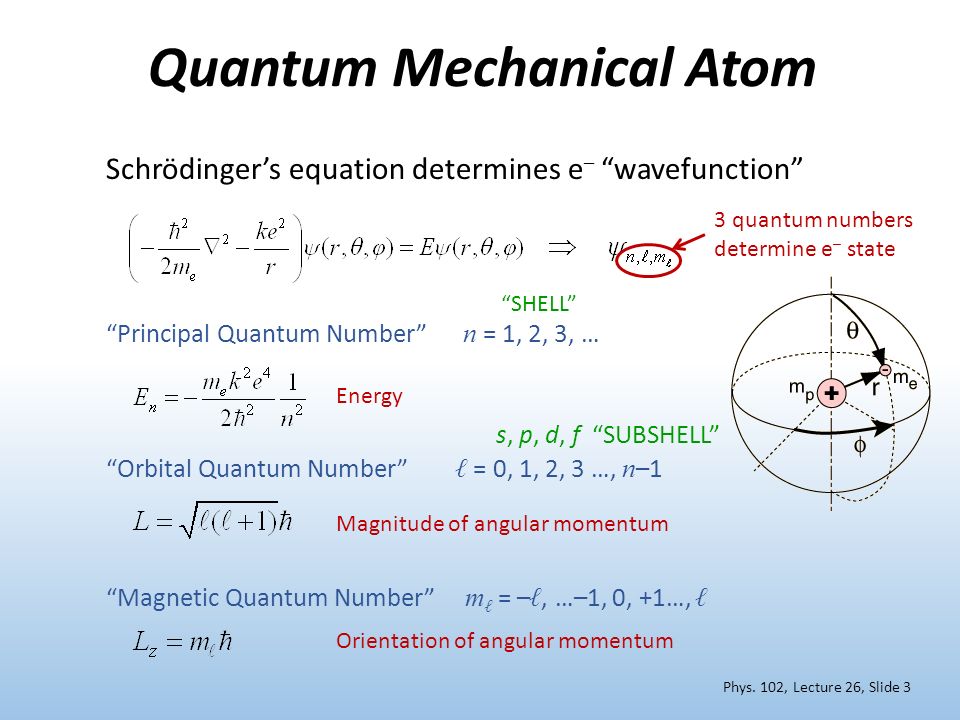
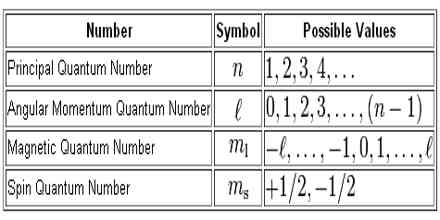
Intrinsic Spin Angular Momentum Is Quantized in Magnitude and Direction. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l) and the electron spin quantum number (m s). There are a total of four quantum numbers;.
Principle Quantum # (definition) Indicates the main energy level occupied by the electron. For electrons, s can only be 1/2, and m s can be either +1/2 or –1/2. 4 For atoms with a well-defined S, the multiplicity of a state is defined as (2S+1).
Angular Momentum Quantum Number. In the pattern of other quantized angular momenta, this gives total angular momentum The resulting fine structure which is observed corresponds to two possibilities for. The possible value of the total spin angular momentum can be found from all the possible orientations of electrons within the atom.
Spin projection m s = +1/2 is referred to as spin up , whereas m s = −1/2 is called spin down. Azimuthal quantum number (l):. The subshell in which the electron can be found is given by the angular momentum quantum number, #l#.
There is only one way in which a sphere (l = 0) can be oriented in space. To learn more about where that elusive electron could be, review the corresponding lesson on Four Quantum Numbers:. However this simplistic picture was quickly realized to be physically impossible, and replace.
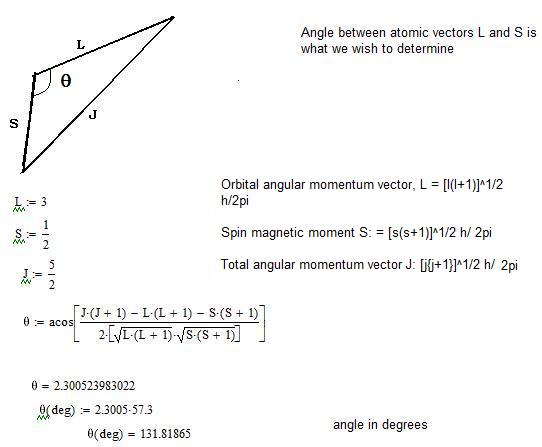
Where the total angular momentum quantum number is. L indicates the shape of the orbital. You have a system of two electrons whose orbital quantum numbers are l 1 = 2 and l 2 = 4 respectively.
Resultant angular momentum number of electron spins. Orbitals have shapes that are best described as spherical (l = 0), polar (l = 1), or cloverleaf (l = 2). The total spin momentum has magnitude Square root of √S(S + 1) (ℏ), in which S is an integer or half an odd integer, depending on whether the number of electrons is even or odd.
It depends on the angular velocity and distribution of mass around the axis of revolution or rotation and is a vector quantity with the. Spin quantum number (s. The spin quantum number indicates the orientation of the intrinsic angular momentum of an electron in an atom.
Quantum numbers often describe specifically the energy levels of electrons in atoms, but other possibilities include angular momentum, spin, etc. ℓ is greater than or equal to zero and less than or equal to n-1. For p, d, and f subshells, two peaks are observed due to a magnetic interaction between the spin of the electron and its orbital angular momentum.
The electron cloud model is an atom model wherein electrons are no longer depicted as particles moving around the nucleus in a fixed orbit. You're thinking about energy levels or shells, and you're also thinking about average distance from the nucleus. The z-component of spin angular momentum is then where.
Spin Quantum Number (ms):. The principal quantum number, n, corresponds to:. The orbital angular momentum operator is the quantum-mechanical counterpart to the classical notion of angular momentum:.
In atomic physics, the spin quantum number is a quantum number that describes the intrinsic angular momentum of a given particle. The possible values of S, the total electron spin angular momentum quantum number, are given by eq. Magnetic quantum number (m l):.
Spin Quantum # (symbol) s. Ms = +½ or -½. The answer is 4.
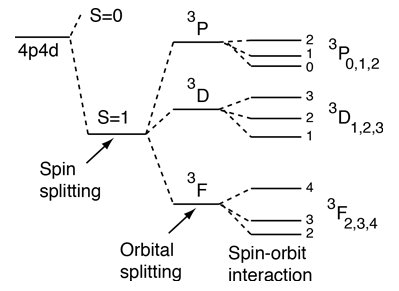
In practice, spin is given as a dimensionless spin quantum number by dividing the spin angular momentum by the reduced Planck constant ħ, which has the same dimensions as angular momentum, although this is not the full computation of this value. The SI unit of spin is the (N·m·s) or (kg·m 2 ·s −1), just as with classical angular momentum. As discussed in Chapter 4, the spin-orbit interaction causes a splitting of these states according to the formula.
A) Yes B) No Question 7 (2 pts) Multiparticle systems are described by linear combinations of the individual wave functions. An electron can spin in only one of two directions (sometimes called up and down). If n = 2, l could be either 0 or 1.
In atoms, there are a total of four quantum numbers:. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms). Here, ℏ is the reduced Planck constant.
Principal, Angular Momentum, Magnetic & Spin Quantum Numbers. Describes the orbital of subshell and magnetic moment. The principal quantum numbers are represented by the letters 'K, L, M.
There are a set of angular momentum quantum numbers associated with the energy states of the atom. This lesson will. For example, in the absence of external fields, the energy eigenstates of Hydrogen (including all the fine structure effects) are also eigenstates of total angular momentum.This almost has to be true if there is spherical symmetry to.
Introduction Angular momentum plays a central role in both classical and quantum mechanics. Principal Quantum Number ‘n’ :The principal. The spin angular momentum is a vector-like quantity and is quantized by the spin quantum number s.
Possible values of S (unitless) = n/2 (3b) From eq. The magnetic quantum number is the orientation of the orbital with integer values ranging from -ℓ to ℓ. Angular Momentum in Quantum Mechanics Asaf Pe’er1 April 19, 18 This part of the course is based on Refs.
The Spin Quantum Number (\(m_s\)) describes the angular momentum of an electron. The spin quantum number is designated by the letter s, and is the fourth of a set of quantum numbers, which completely describe the quantum state of an electron. Absolute values of the projection of the resultant orbital angular momentum on the molecular axis.
Σ is the projection of S on the molecular axis. This is equal to the number of different possible values of the total (orbital plus spin) angular momentum J for a given (L, S) combination, provided that S ≤ L (the typical case). Consequently, the restriction that the quantum number of the overall angular momentum must take integer values does not apply to spin angular momentum, because this restriction (found in Sections 4.3 and 4.4) ultimately depends on Equations -.
Both were first discovered for electrons in conjunction with fine structure in atomic spectra. Total Angular Momentum When the orbital angular momentum and spin angular momentum are coupled, the total angular momentum is of the general form for quantized angular momentum. 3a and 3b we deduce that the total spin quantum number may be equal to 0, 1/2, 1, 3/2, 2, etc.
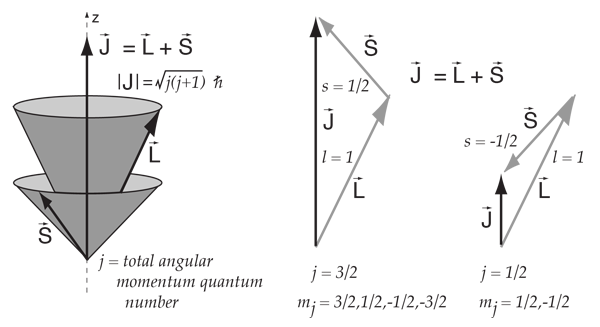
Which electron spin state has the lower energy in the absence of an external magnetic field?. The spin and orbital angular momentum states of any particle with spin s = 1/2 and orbital angular momentum l > 0 can be combined to form states with the total angular momentum quantum number j = l ± 1/2. The angular momentum quantum number is symbolized by l.
S z is the z-component of spin angular momentum and m s is the spin projection quantum number. Principal quantum number angular momentum quantum number. The angular momentum quantum number, signified as (l), describes the general shape or region an electron occupies—its orbital shape.
Addition of Angular Momentum. An important family is flavour quantum numbers – internal quantum numbers which determine the type of a particle and its interactions with other particles through the fundamental forces. Assuming n values are correct, the incorrect set of quantum numbers are:.
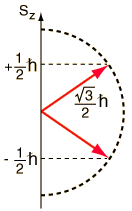
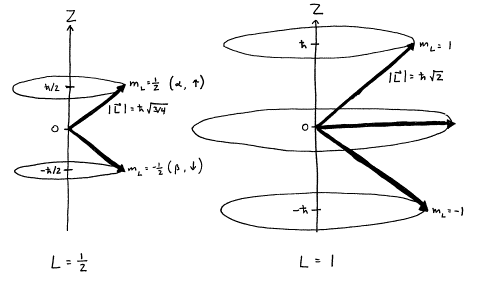
Given an arbitrary direction z (usually determined by an external magnetic field) the spin z-projection is given by where m s is the secondary spin quantum number, ranging from −s to +s in steps of one. Specifies the orientation of the spin axis of an electron. And that the magnitude of the spin angular.
For an electron located on the second energy level, there are only two possible. Quantum numbers are used to describe the probable location of the electron in one atom. It describes the quantum state of an electron, including its energy, orbital shape, and orbital orientation.
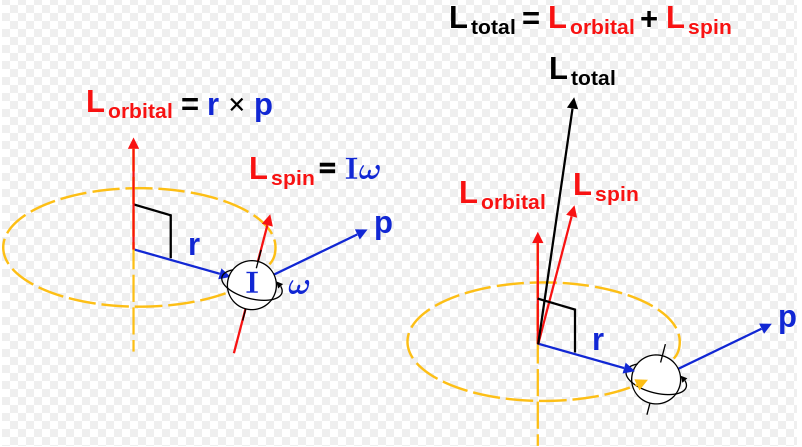
There are two more quantum numbers of immediate concern. They can even take on more complex shapes as the value of the angular quantum number becomes larger. The spin angular momentum associated with electron spin is independent of orbital angular momentum, which is associated with the electron's journey around the nucleus.
The angular quantum number (l) describes the shape of the orbital. This gives a z-component of angular momentum. The principal quantum number 'n' represents orbit number hence, determine the size of orbitals.
Start studying Quantum Numbers. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The positive value of msimplies an upward spin on the electron which is also called ‘spin up’ and is denoted by the symbol ↑.
It arises when a particle executes a rotating or twisting trajectory (such as when an electron orbits a nucleus). This intrinsic value of angular momentum gives rise to the magnetic moment possessed by the electron and in turn, this magnetic moment accounts for the presence of a subatomic magnetic phenomenon. Electron spin is not used to define electron shells, subshells, or orbitals, unlike the quantum numbers n, l, and m l.
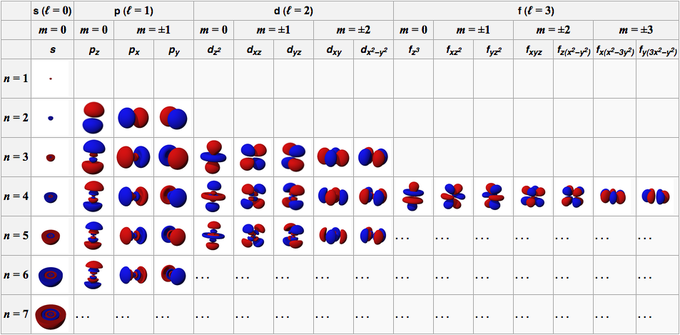
The angular momentum quantum number is an integer that is the value of the electron's orbital (for example, s=0, p=1). The value of l depends on the value of the principle quantum number n. Quantum number for vibrational angular momentum.
Notice that #l# can take values from #0# to #n-1#. This question has multiple correct options. This kind of coupling gives an even number of angular momentum levels, which is consistent with the multiplets seen in.
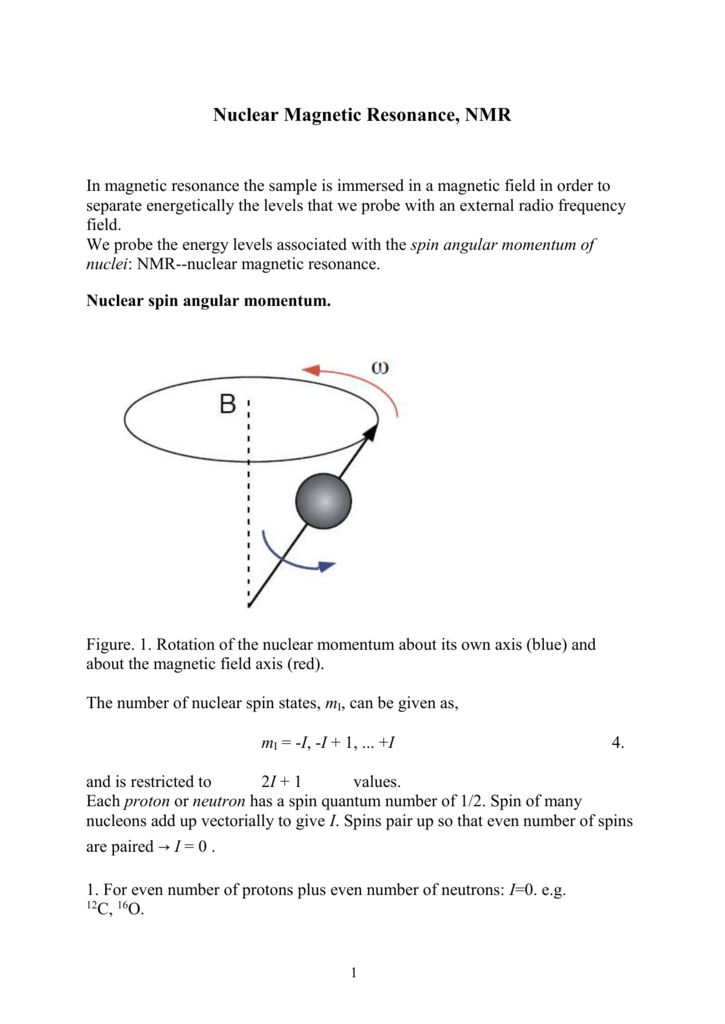
The only possible values of a spin quantum number are +½ or -½ (sometimes referred to as 'spin up' and 'spin down'). I or (I i ) Angular momentum quantum number of nuclear spin for one (or i th) nucleus. Angular momentum quantum numbers.
Don't worry, nobody understands these in first-year chemistry. Here L is the total orbital angular momentum quantum number. In other words, the spin quantum number is.
This will tell us the shape of the orbital. The energy level of the electron. (b) Find the possible values of s (total spin angular momentum quantum number) for the system.
To describe the state of an electron in an atom/ion, there are 4 quantum numbers. Spin is one of two types of angular momentum in quantum mechanics, the other being orbital angular momentum. Question 6 (2 pts) The spin s is the quantum number which quantizes the z-component of the particle's intrinsic angular momentum?.
There are two 3 s electrons and two 3 p electrons. Magnetic Quantum # (symbol) m. Now, the energy level is given by the principal quantum number, #n#, which in this case is equal to #2#.
3b, where n is 0 or a positive integer. The possible values of the electron spin quantum number are +½ and -½. You just pretend to, and then in second-year you learn them.
The Angular Momentum Quantum Number, represented by the letter l, is also called the Orbital Quantum Number because it determines the path or area that the electron travels within, which we define as an orbital in chemistry. The spin angular momentum is given by where is the spin quantum number and is h-bar. The value of msoffers insight into the direction in which the electron is spinning.
A set of quantum numbers are given for paired electrons or for one electron in an orbital. Because angular momentum is a vector, the Spin Quantum Number (s) has both a magnitude (1/2) and direction (+ or -). Provides information about subshell and angular momentum.
All start with 3, so all will have a principal quantum number of 3. The name comes from a physical spinning of the electron about an axis that was proposed by Uhlenbeck and Goudsmit. 1 QUANTUM NUMBERS We have assumed circular orbits Then for hydrogen eV n En 13.6 1 2 and 2 h L n If you know n you know both energy and angular momentum Only one quantum number.
Ms=+12 and ms=−12 have the same energy. Spin "up" and "down" allows two electrons for each set of spatial quantum numbers.:. The “n ” term represents the shell, “ l ” the subshell, and “ j ” the total angular momentum.
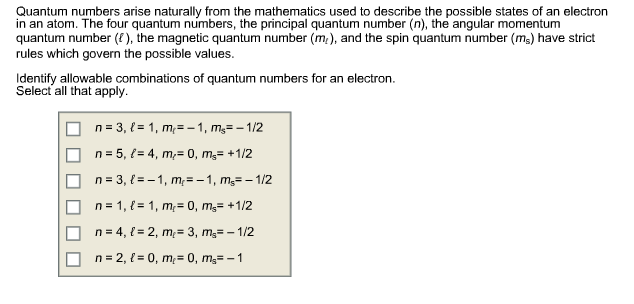
Solved Quantum Numbers Arise Naturally From The Mathemati Chegg Com

Four Quantum Numbers Principal Angular Momentum Magnetic Spin Video Lesson Transcript Study Com
Q Tbn 3aand9gcr1wdicsobbl1pn Rljj Kuleotfsbszg7vblhzzdf7zy4mtrbn Usqp Cau
Spin Angular Momentum Quantum Number のギャラリー
Www Chem Tamu Edu Rgroup Marcetta Chem362 Lectures 362 lec 4 and 5 spring 17 term symbols zeff and periodic prop Pdf
What If There Are 3 Unpaired Electrons What Would Be Its Multiplicity Quora

Atomic And Molecular Quantum Numbers Astrobaki

Angular Momentum Operator Wikipedia
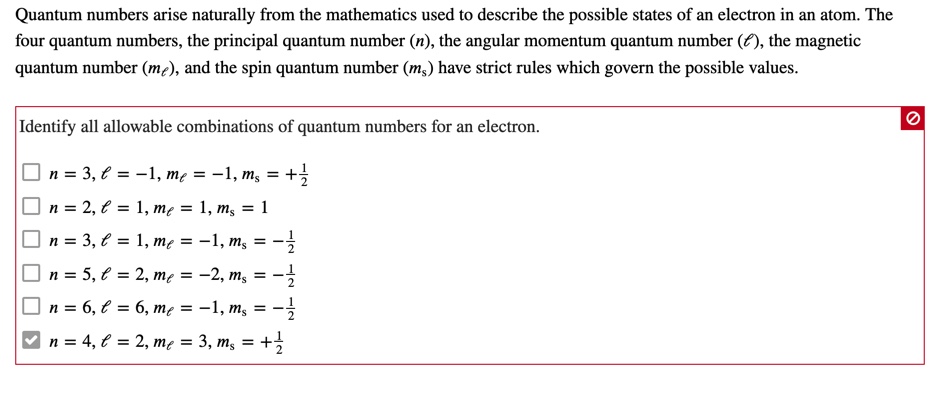
Answered Quantum Numbers Arise Naturally From Bartleby
Spin Questions And Answers In Mri
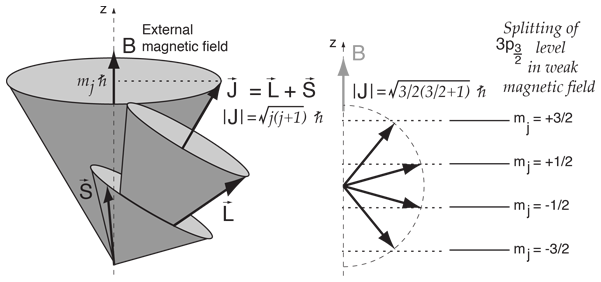
Vector Model Of Angular Momentum
Electron Spin
Brane Space Spin Orbit Coupling In Quantum Mechanics

Physics Ch 66 5 Quantum Mechanics The Hydrogen Atom 36 Of 78 Spin Angular Momentum Youtube

Principle Quantum Number N 1 2 3 Describes Orbital Size And Energy Angular Momentum Quantum Number L 0 To N 1 Describes Orbital Shape Magnetic Ppt Download

Angular Momentum Quantum Number Definition Example Video Lesson Transcript Study Com

Spin Quantum Number An Overview Sciencedirect Topics
Http Www Cabrillo Edu Jmccullough Physics4c Files Ch40 Pdf
Physical Principles Of Nmr Spectroscopy
Solved Quantum Numbers Arise Naturally From The Mathemati Chegg Com

Curvilinear Motions In Newtonian Mechanics And Quantum Spin

Chapter 7 Atoms In A Magnetic Field Ppt Download
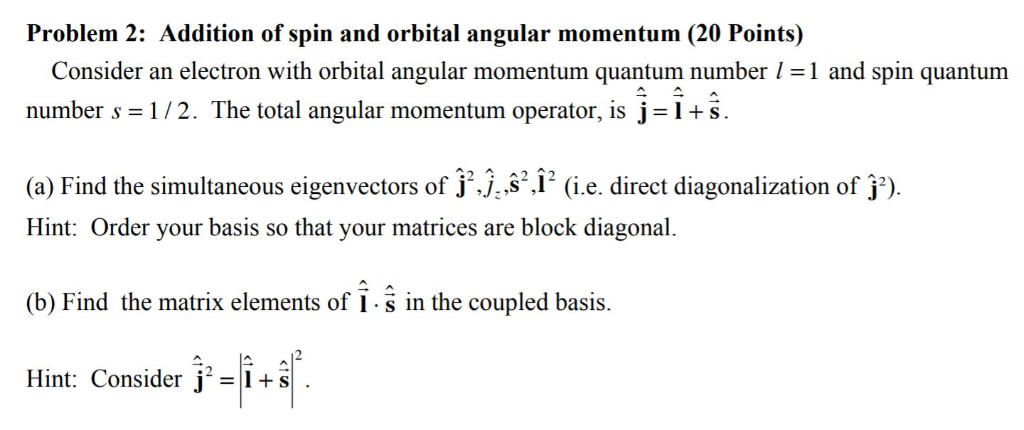
Solved Problem 2 Addition Of Spin And Orbital Angular Mo Chegg Com

The Angular Momentum Quantum Number L Youtube
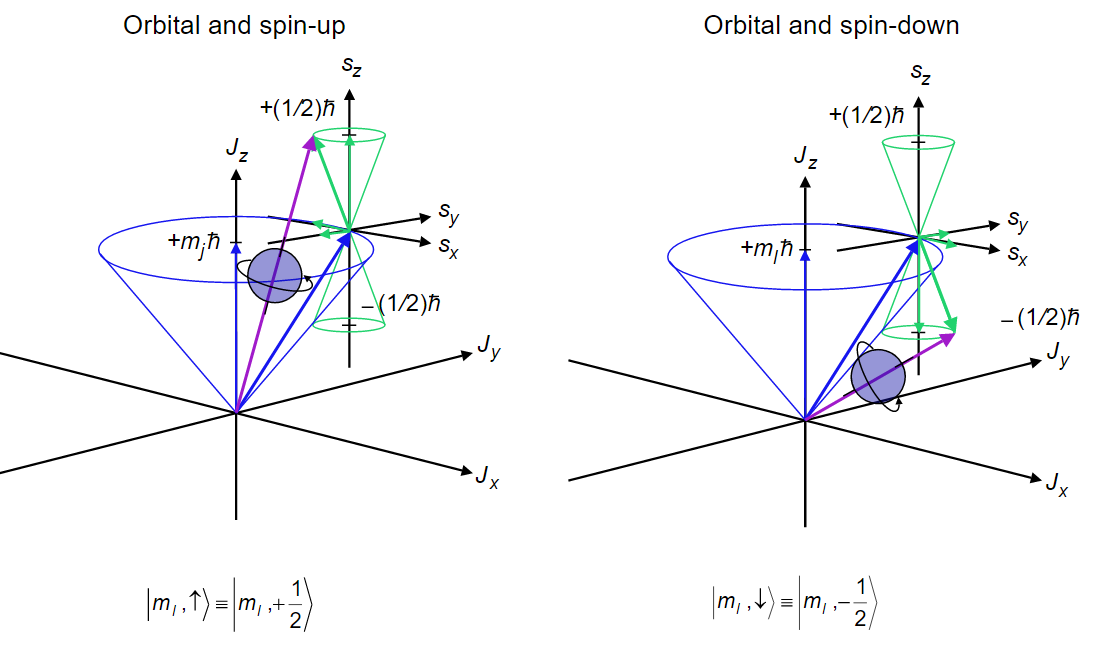
8 9 The Allowed Values Of J The Total Angular Momentum Quantum Number Chemistry Libretexts
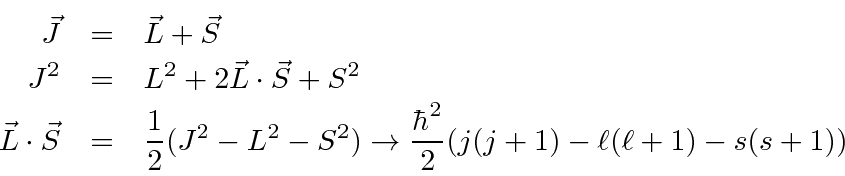
Total Angular Momentum And The Spin Orbit Interaction

Oneclass Quantum Numbers Arise Naturally From The Mathematics Used To Describe The Possible States O
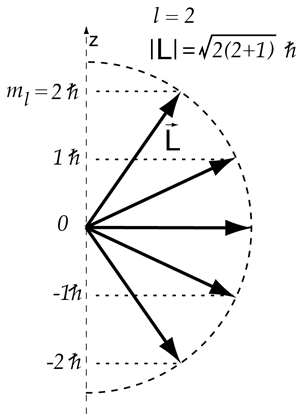
Vector Model Of Angular Momentum

Quantum Numbers Video Quantum Physics Khan Academy

Angular Momentum Quantum Numbers L Quantumnumbersprojectterm1
What Does The Quantum Number 1 2 1 2 For An Electron Spin Tell Does It Tell Direction Of Spin If Yes Then What Direction Quora
2

Quantum Number Symbols On Keyboard

Spin And Addition Of Angular Momentum Ppt Video Online Download

Pdf Spin And Orbital Angular Momentum Of Photons

Quantum Angular Momentum
Quantum Numbers And Atomic Energy Levels
Quantum Numbers Introduction To Chemistry
Solved Quantum Numbers Arise Naturally From The Mathemati Chegg Com

Quantum Number Wikipedia

Solved For Two Electrons The Z Component Of The Total Orb Chegg Com
What Are The Quantum Numbers Of The Five Electrons Of Boron Socratic

10 Electron Spin Angular Momentum Coupling
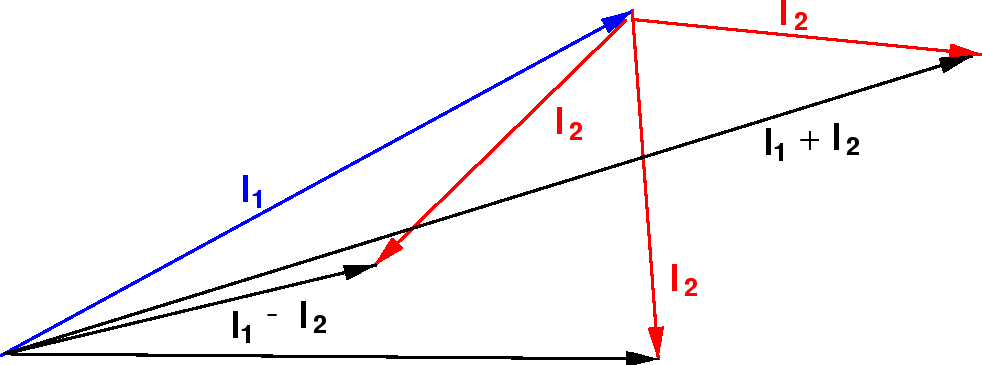
Addition Of Angular Momentum

Openstax College Physics Solution Chapter 30 Problem 49 Problems Exercises Openstax College Physics Answers
Quantum Numbers To Periodic Tables Chemogenesis
Phys 102 Lecture 26 The Quantum Numbers And Spin Ppt Video Online Download

Solutions 13
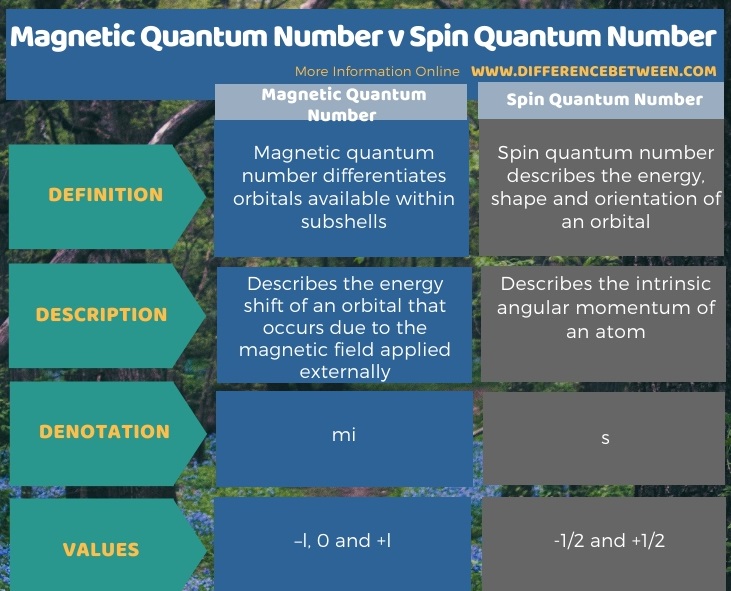
Difference Between Magnetic Quantum Number And Spin Quantum Number Compare The Difference Between Similar Terms

Quantum Numbers Atomic Orbitals And Electron Configurations
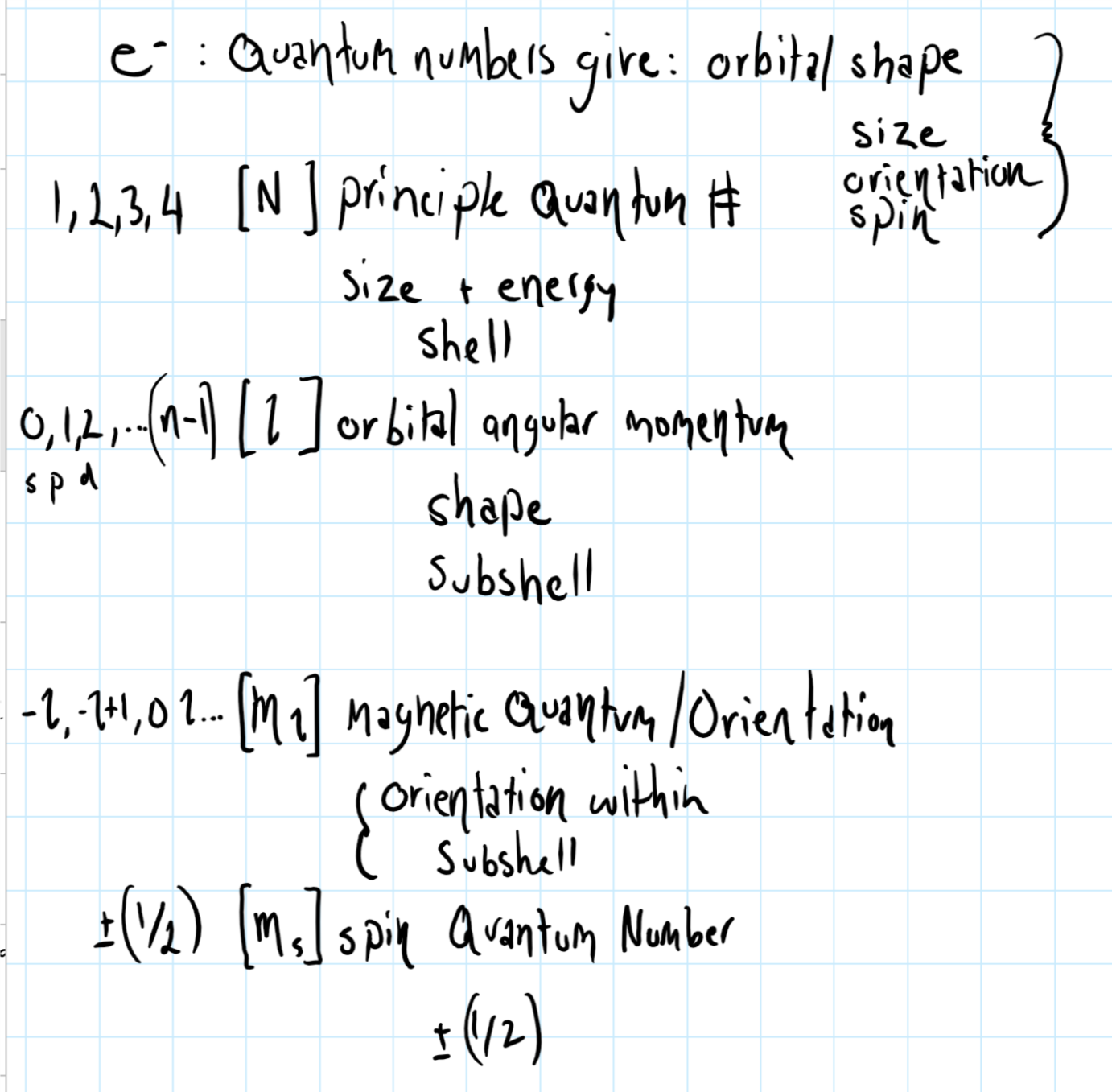
What Do The Four Quantum Numbers Describe About An Electron Socratic

Quantum Angular Momentum

Angular Momentum Quantum Number Shapes Quantum Momentum Tools For Teaching
Vector Model Of Angular Momentum
Q Tbn 3aand9gcsxlrns1yue33e8oq6jm5q71qpbvjo9yrclbnkyptvw5n2ncmz6 Usqp Cau
8 8 Term Symbols Gives A Detailed Description Of An Electron Configuration Chemistry Libretexts
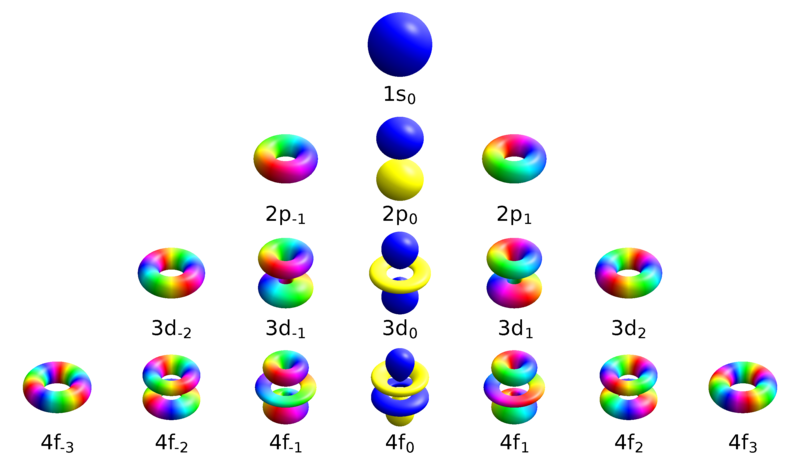
Difference Between Magnetic Quantum Number And Spin Quantum Number Compare The Difference Between Similar Terms
The Angular Momentum And The Spin Of A Particle Fair Science
Q Tbn 3aand9gcting5kfz8 N3jbq3tfb3iy0 Jrdzsk9t17afb8ehrveouvclzt Usqp Cau
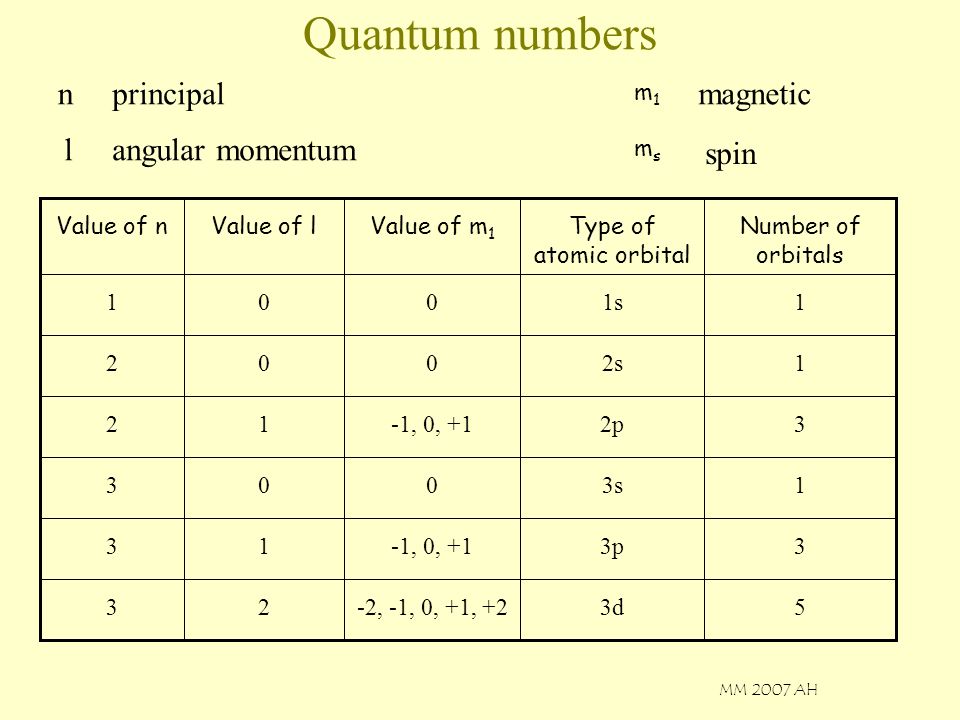
Quantum Numbers N Principal Magnetic L Angular Momentum Spin M1 Ms Ppt Video Online Download
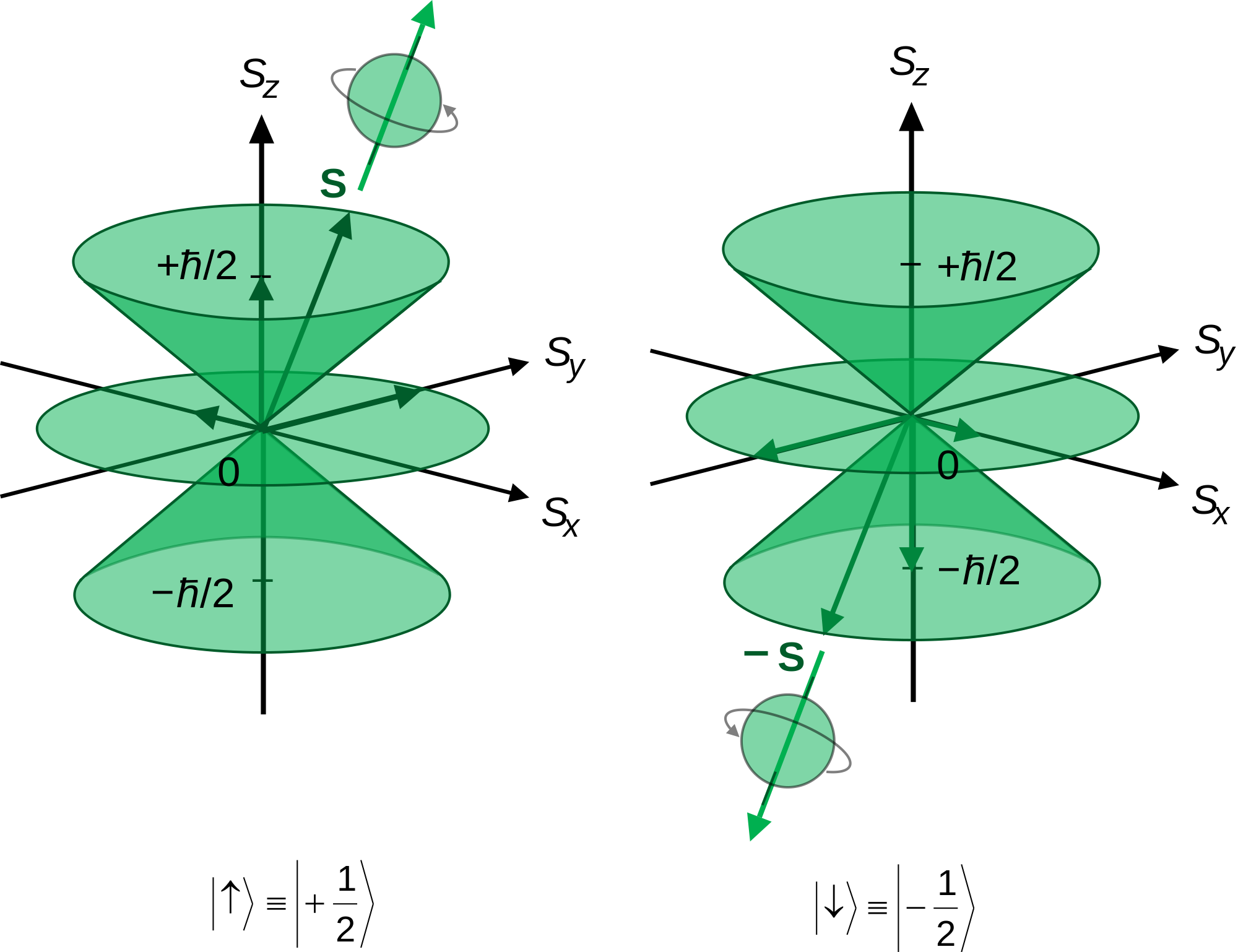
Does The Angular Momentum Quantum Number L Designate The Shape Of The Orbital Socratic

Total Logo Angular Momentum Operator Rotation Operator Spin Quantum Mechanics Translation Total Angular Momentum Quantum Number Hamiltonian Angular Momentum Operator Operator Momentum Operator Png Pngwing
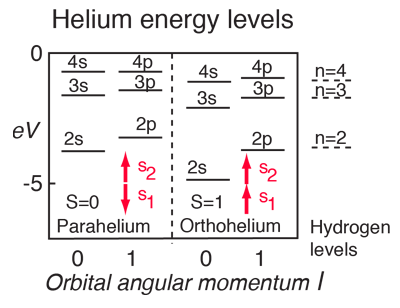
Quantum Numbers And Atomic Energy Levels
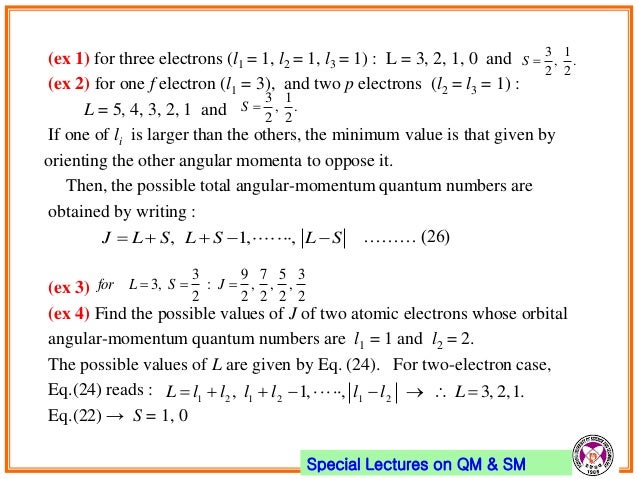
10 Electron Spin Angular Momentum Coupling

Quantum Numbers State Multiplicity Specifically Concerned With The Differences Between L L And S S Physics Stack Exchange
Web Docs Gsi De Wolle Telekolleg Kern Lecture Wollersheim 16 Iit Ropar Nuclearphysics 6 Nuclearangularmomentum Pdf

Consider The He Atom It Has 2 Electrons Each With Its Own Spin And Adding Spin Angular Momenta Means Adding Vectors With This In Mind What Are The Ppt Download
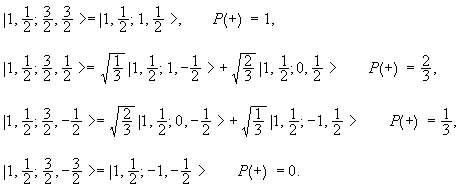
Addition

Solved To Understand And Be Able To Use The Rules For Det Chegg Com
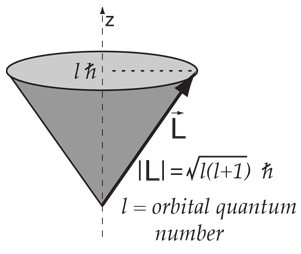
Vector Model Of Angular Momentum
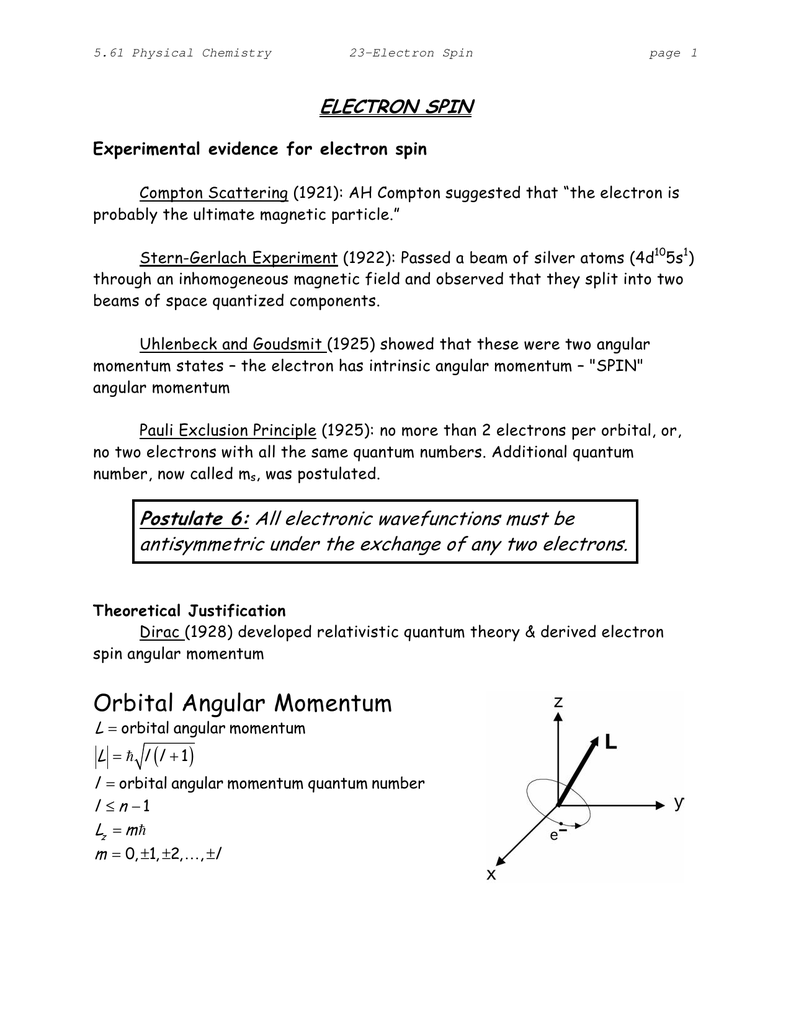
Document
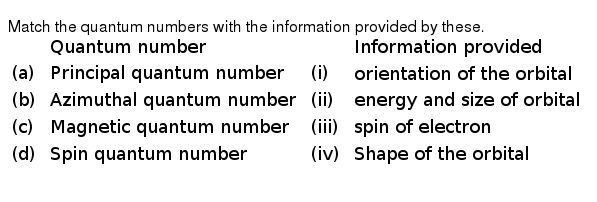
What Does The Angular Momentum Quantum Number Determine Check Th

Chm 261 Quiz 9 Chm 261 General Chemistry I Csu Studocu

Quantum Numbers And Rules Physics

Quantum Number

Total Angular Momentum An Overview Sciencedirect Topics

10 Electron Spin Angular Momentum Coupling
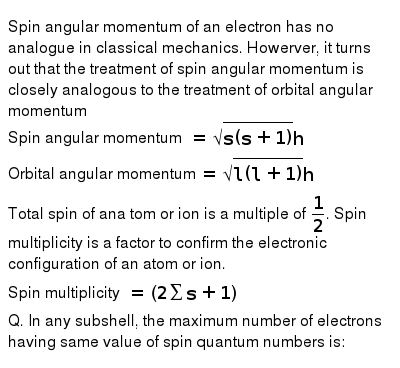
Spin Angular Momentum Of An Electron Has No Analogue In Classical
What Is The Formula For Spin Angular Momentum Quora

Openstax College Physics Solution Chapter 30 Problem 41 Problems Exercises Openstax College Physics Answers

Spin Quantum Number Definition Example Video Lesson Transcript Study Com
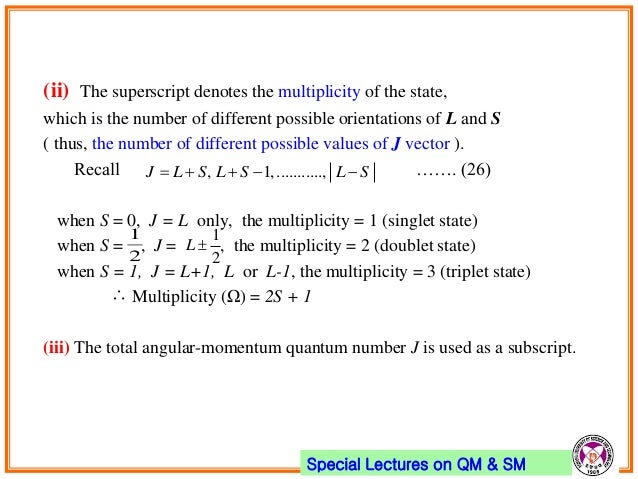
10 Electron Spin Angular Momentum Coupling

Quantum Mathematics 34 4 Operators For Spin Angular Momentum Youtube

Physics Ch 66 5 Quantum Mechanics The Hydrogen Atom 45 Of 78 Angular Momentum Vector J Youtube
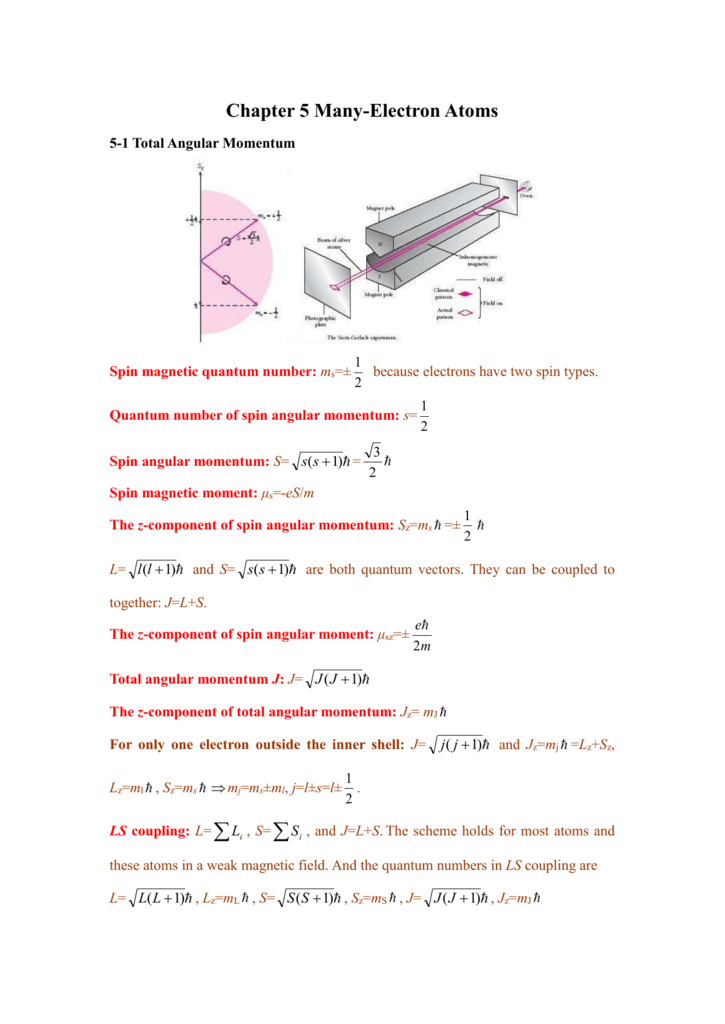
Chapter 5 Many Electron Atoms 5 1 Total Angular Momentum Spin
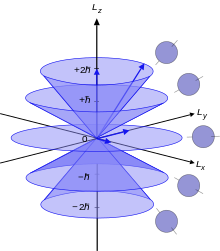
Azimuthal Quantum Number Wikipedia
What Are Quantum Numbers Qs Study

Quantum Numbers And Rules Physics

Quantum Angular Momentum

Oneclass Quantum Numbers Arise Naturally From The Mathematics Used To Describe The Possible States O

Angular Momentum Quantum Number Definition Example Video Lesson Transcript Study Com
Angular Momentum Azimuthal Quantum Number Rotation Spin Png 1280x1013px Angular Momentum Angular Velocity Area Atomic Orbital
Spin And Addition Of Angular Momentum Ppt Video Online Download
Nuclear Magnetic Resonance Nmr

Section 2 Atomic Spectra Lectures 2 3 Ish Pdf Free Download
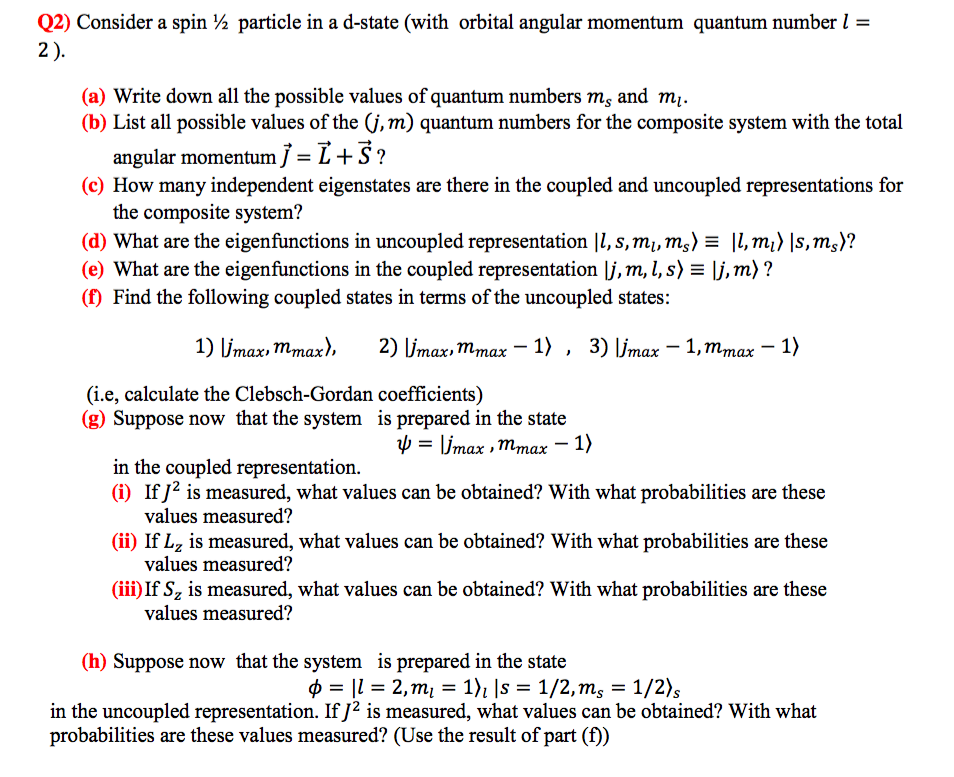
Solved Q2 Consider A Spin 2 Particle In A D State Wit Chegg Com

Quantum Numbers And Rules Physics
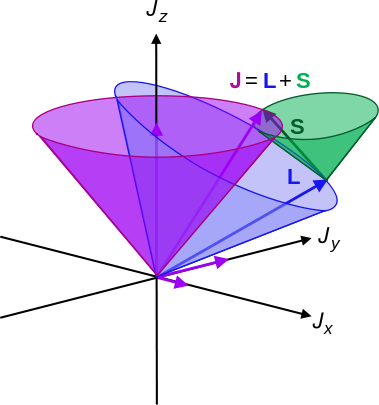
8 9 The Allowed Values Of J The Total Angular Momentum Quantum Number Chemistry Libretexts
10 Electron Spin Angular Momentum Coupling

Spin Quantum Number Chemistrygod
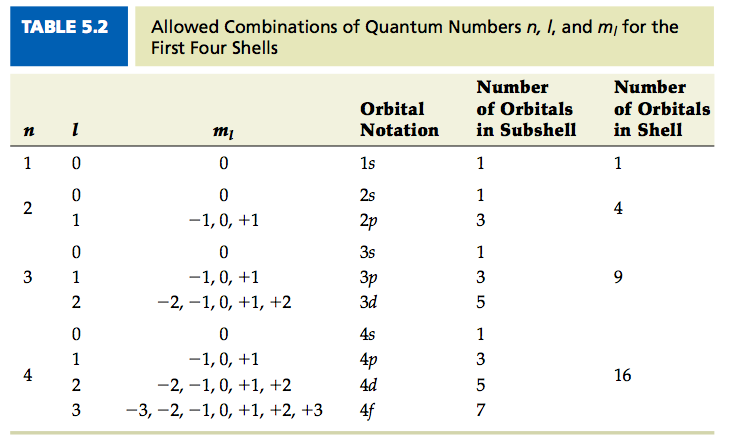
Quantum Numbers Chemistry
Q Tbn 3aand9gcsxlrns1yue33e8oq6jm5q71qpbvjo9yrclbnkyptvw5n2ncmz6 Usqp Cau
Angular Momentum Quantum Knowino

Quantum Angular Momentum

Total Angular Momentum Quantum Number Youtube



